Multi-PCBA Test Solution Delivers Broad Functional Test Coverage for FDA Compliance
- Nov 4, 2022
- 4 min read
Updated: Aug 8
A medical device manufacturer required a complete functional test solution consolidating their product verification test phase and quality control process for their FDA-compliant product.
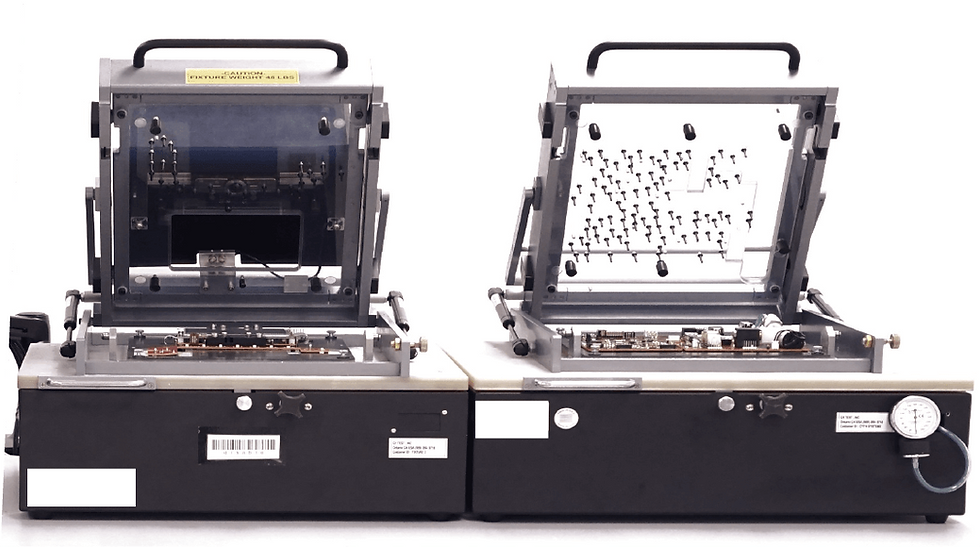
Project Summary
Cyth developed a unified test platform using PXI, LabVIEW, and TestStand spanning test plans, fixturing, and a common automation architecture for six PCBAs used in patient monitoring devices.
System Features & Components
PXI instrumentation mapped to multi-DUT test coverage
Custom fixturing for 6 constituent PCBAs, including bed-of-nails connectivity to test points
RF environmental enclosure for wireless test
Measurement software (LabVIEW) and test automation executive (TestStand) with common architecture for efficient reuse across devices-under-test (DUTs)
Outcomes
Minimized capital costs through instrumentation configurations shared across devices-under-test
Reduced engineering development and maintenance effort with common measuremeent code and test automation software
Successfully achieved schedule milestones for factory acceptance testing (FAT)
Technology at-a-glance
PXIe Chassis: PXIe-1078
Data acquisition: PXI-6229
Sensors and signal conditioning: pressure transducer, digital signal attenuator, programmable DC loads
Power: PXIe-4112 and PXIe 4113 programmable power supplies
Switching: PXI-2564 SPST relay module and PXI-2534 matrix switch
Serial communication: PXI-8432 RS232 interface module
Environmental: RF Faraday enclosure
Test automation software: LabVIEW and TestStand
Enabling Responsive Patient Care
Vital signs monitoring equipment is the quiet workhorse of patient care. These systems take critical physiological measurements, such as temperature, respiratory rate, blood oxygenation, and blood pressure to provide clinicians with quantifiable data to assess patient health status and automatically detect changes that may indicate emergencies requiring active intervention.
Like many medical devices and hospital equipment, modern vital signs monitors have evolved from simple measurement devices to sophisticated digital systems capable of continuous monitoring, wireless data transmission, and integration with electronic health records (EHRs), enabling more responsive patient care and improved clinical outcomes. While these products have become more intelligent and automated, the need for rigorous testing across design and production phases continues to grow.
Enhanced Visibility & Monitoring
The product incorporates four vital measurements across two distinct measurement subsystems:
Cuff attached to the patient’s arm – measures systolic and diastolic blood pressure
Pulse oximeter clipped to the patient’s finger – measures oxygen levels, heart rate, and temperature
These subsystems are physically connected back to a processing and display unit. The system is also capable of transmitting patient readings wirelessly and securley to local devices, such as a nurse’s tablet, for enhanced visibility and monitoring.

Comprehensive Test Coverage
The engineering team responsible for testing the product faced several core challenges:
Finding a test solution that would provide comprehensive test coverage for six individual PCBAs and final assembly verificcation
RF testing for wirless connecitivty in a controlled, interference-free environment
Reproducible and reliable test data for FDA approvel and ongoing compliance requirements
They were looking for outside help from an engineering firm experienced with PCBA and final assembly functional test, ranging from fixture design, instrumentation selection and connecivity, to test software development.
Designing a Unified Test Platform
After engaging with the client on the product's test requirements, budget, and timeline, our engineering team started by mapping the intended test coverage to instrumentation capable of performing the measurements. We then recognized an opportunity to optimize footprint and overall hardware utilization by consolidating the various DUTs into three bed-of-nails fixtures and two test enclosures. This allowed us to refine the hardware BOM, lowering overall cost spread across the DUTs.
Instrumentation Selection:
PXIe Chassis: PXIe-1078
Data acquisition: PXI-6229
Power: PXIe-4112 and PXIe-4113 programmable power supplies
Switching: PXI-2564 SPST relay module and PXI-2534 matrix switch
Serial communication: PXI-8432 RS232 interface module

After finalizing the instrumentation BOM, our team planned out the signal routing diagram, including strategic switching, from the PXI modules' channels all the way to the DUTs' test points, as accessed through cabling and the fixtures' pogo pins. Throughout this process, we leveraged our PCBACheck reference design for advanced starting points on fixture CAD, layout, and sub-components.
In effect, this versatile test fixture design allowed for multiple DUTs to be tested simultaneously, increasing the throughput and efficiency of the system without requiring additional instrumentation.

Environmental RF Test
One of the more complex challenges involved validating the product's wireless interface. To do so, we needed to create a controlled environment free of RF noise and interference, opting to design in an RF Faraday enclosure to create such an environment.
We developed a comprehensive test protocol that exercised the wireless PCBA across multiple power modes using a 3-bit control signal. Through a digital signal attenuator, we measured transmission power and signal quality across the ISM Band (Industrial, Scientific, and Medical), specifically at 838 MHz and 916 MHz frequencies. This test methodology supported the project requirements of:
Validating signal strength at various Tx/Rx distances
Verifying proper power mode transitions
Documenting signal quality metrics for regulatory compliance
Test Software & Regulatory Compliance:
Our engineering team developed the test software in conjunction with the hardware design and build aspects of the project. Using our PCBACheck software framework, we developed individual test modules in LabVIEW using a hardware abstraction layer in the form of driver APIs and pre-existing measurement expertise. From there, we incorporated those discrete, reusable code modules into multiple TestStand sequences capable of executing the test plan for each individual DUT. TestStand, and the PCBACheck automation framework for functional test provides the following benefits:
Test sequence development – graphical sequence editor for code module integration and validation against test requirements
Test execution – flexible process models and multi-threaded resource management
Data access – customizable operator interfaces, report generation, and database connectivity

Implementing a unified test automation framework across the DUTs helped with code reuse, debugging, and test data repeatability. Having reliable, consistently formatted test data helped our client with their Factory Acceptance Test (FAT) phase and lowered the effort to collect and report on compliance metrics for the FDA, avoiding many hidden costs and unpredictable headaches.

Full Turnkey Test Coverage
Overall, the Cyth team delivered a complete turnkey solution of two PCBA functional test enclosures and a final assembly test for our client's patient monitoring product within schedule and budget. The unified test platform provided a versatile fixture design and intelligent instrumentation routing, helping control capital equipment costs. The test automation software provided full test coverage for the various individual DUTs, making test data easily accessible and repeatable. In effect, this solution helped our client spin up their factory test capabilities sooner than an in-house approach, clearing the product's path to market and easing downstream quality control efforts.





Comments